
With multiple patients being evaluated using durable medical devices like MRI machines on a daily basis, keeping equipment clean is an important part of keeping patients healthy. Product compatibility between these disinfectants and the materials used on the medical device surfaces are critical.
In November 2019, a simple sample request between PDI and SEKISUI KYDEX, two leading manufacturers for the medical community, evolved into a testing opportunity to demonstrate the efficacy of PDI disinfectants and KYDEX® Thermoplastics.
PDI is a global leader in the fight against preventable infections. It is their goal to manufacture disinfectants that are strong enough to eliminate harmful bacteria, yet gentle enough to preserve the integrity of the medical devices that are being disinfected.
SEKISUI KYDEX innovates and creates sustainable thermoplastic material solutions for the next generation of product design. KYDEX® Thermoplastics are durable, chemical resistant, inherently antimicrobial, and will not be stained by harsh cleaning agents, making them ideal for medical devices.
“For years, PDI has been innovating its disinfecting product line to ensure we continue to provide products that meet today’s clinical efficacy needs, yet are gentle enough to preserve the integrity of the medical devices being disinfected,” said Candice Taylor, Senior Director Product Development. She continued, “Our collaboration with SEKISUI KYDEX for testing has helped ensure we are collectively building compatibility into the development process, ultimately helping healthcare facilities maintain the physical integrity of the medical device that are disinfected and reducing the risk of infection transmission.”
THERMOPLASTIC AND DISINFECTANT COMPATIBILITY
Many plastics used for medical devices suffer from environmental stress cracking from exposure to healthcare-grade disinfectants. Plastic degradation can lead to loss of device function and eventually complete device failure. Understanding thermoplastic limits of chemical resistance to healthcare grade disinfectants is a critical step in material selection by design engineers to ensure medical devices meet their intended product service life.
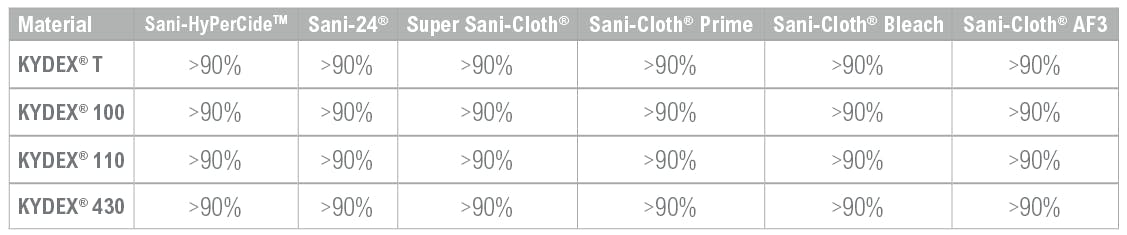
PDI and SEKISUI KYDEX set out to test the possible degradation of KYDEX® Thermoplastics when exposed to PDI medical-grade disinfectants. Testing completed by both PDI and SEKISUI KYDEX labs revealed that KYDEX® Thermoplastics hold up to PDI disinfection products with over 90% mechanical property retention when tested to the ASTM-D543 standard.
Extensive testing was done using the ASTM-D543-20, a globally recognized standard practice for evaluating the resistance of plastics to chemical reagents. Materials were tested using both immersion testing and reagent exposure testing. Weight, dimension changes, and mechanical properties were evaluated for material degradation.
To perform the initial testing, CNC routed KYDEX® thermoplastics tensile bars were used. Thermoplastic products tested included KYDEX® T, 100, 110, 430. Thermoplastics were exposed to Sani-HyPerCideTM, Sani-24®, Super Sani-Cloth®, Sani-Cloth® Prime, Sani- Cloth® Bleach, Sani-Cloth® AF3 disinfectants from PDI.
“What better way to prove how our products hold up to the chemical disinfection process than by collaborating with PDI, an industry leader in healthcare wipes? Recognized for their Sani-Cloth® brand, they are a household name in the healthcare industry,” says Mark Denning, SEKISUI KYDEX Medical Market Business Manager. “They are a great industry leader to partner with on our current and future product offerings.”
ENVIRONMENTAL STRESS CRACKING
Environmental stress cracking testing was guided by ASTM-D543. KYDEX® Thermoplastic tensile bars were placed in the strain jig at 1.5% nominal strain. The tensile bars were exposed to PDI disinfectant wipes for 48 hours, at 24-hour intervals the wipes were reapplied. The tensile bars were removed from the jig and allowed to relax for 1hr or greater. These tensile bar sets were later used to evaluate changes in the material’s tensile properties. A more rigorous set of tests were conducted by extending the exposure periods. SEKISUI KYDEX extended the exposure testing to a full 28 days, in an effort to force a material failure.
MECHANICAL PROPERTY RETENTION
The KYDEX® Thermoplastics tensile bars were removed from the stain jigs after the 48 hour exposure period and one-hour relaxation period to be tested for changes in mechanical properties. Specifically, the bars were tested for changes in tensile stress at yield by via a mechanical tester. Tensile testing was guided by ASTM-D638. Mechanical retention, the quotient of tensile stress at yield for exposed samples compared to the tensile stress at yield for unexposed samples, was calculated. An acceptable test result is 70% mechanical property retention. KYDEX® Thermoplastics retained greater than 90% of its mechanical properties when exposed to PDI germicidal disinfectants.
IMMERSION OBSERVATIONS
Immersion testing measures weight and dimension changes to the thermoplastics when exposed to PDI disinfectants. The test takes a 2” by 2” square submerged in the solution for up to 128 hours, stirring every 24 hours. The material is observed for loss of gloss, developed texture, decomposition, discoloration, swelling, clouding, tackiness, rubberiness, crazing, bubbling, cracking, or solubility.
FUTURE PRODUCT COMPATIBILITY
As a result of the changing landscape for chemical technologies and medical device engineering as a result of the COVID-19 pandemic, PDI and SEKISUI KYDEX are expanding their testing for product compatibility. Currently, testing has evolved to include geometric and formed parts, and new chemical consistencies and technologies. PDI recently acquired Tru-D® SmartUVC, a pioneer in the UVC disinfection industry, and maker of the first UVC robot.
Along with SEKISUI KYDEX, they have begun testing this new disinfection technology with KYDEX® Thermoplastic material, with results expected over the next few months. Additional testing using PDI medical-grade disinfectants will be done on Infused Imaging™ Technology and KYDEX® Injection Molding Resins.
“PDI’s expertise in disinfection and material testing has been a great compliment to the research of our appLab™. With their focus on expanding into other disinfection technologies, such as UV-C, they have obvious plans to stay at the forefront of their industry’s solutions.
“Working with a leader like PDI can help SEKISUI KYDEX understand the future trends of disinfection and what challenges the industry will be facing,” Denning said.
The combination of the efficacy of PDI disinfectants and the durability and chemical resistance of KYDEX® Thermoplastics means that clinicians and medical device designers can rest assured that device design and infection prevention are optimized through this compelling and unique collaboration.





